How do chemical bonds work?
To understand the types of chemical bonding, we should first consider what the atoms of our universe are made of. To do this, we can use a simplified analogy of atomic structure by picturing an atom as a star being orbited by planets. The nucleus is the star, which is formed of positively charged protons and neutrons that have no charge. Negatively charged electrons are the orbiting planets. In an atom’s native form the number of electrons and protons are equal, which gives the atom a neutral charge overall. But other atoms are also attracted to these electrons, and when they’re shared or stolen, a chemical bond is the result.
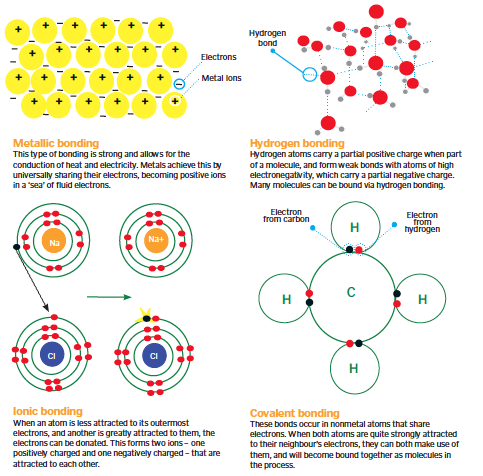
Types of Bonding
How chemicals are bound together is largely dependent on how they share their electrons.
For more information about science and technology, visit our website now. If you have a tablet or smartphone, you can also download the latest digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!
Why are some chemicals more reactive than others?
The wonders of water: How is this chemical the key to life?
Coffee chemistry: Why does your morning hit of caffeine taste so good?





