How glass is made
by Ailsa Harvey · 21/01/2021
From its ancient origins to modern-day technology, what makes this material so versatile?

It’s usually encountered as a transparent pane, so it is easy to look past – or right through – glass. But have you ever stopped to think not about the view beyond your window, but of the window itself? This impressively clear, firm material before you is just one of many variations of glass. Glass can be transparent or opaque, coloured or clear, bullet-stoppingly thick or wafer thin. Every day we use it, whether we drink from a bottle made out of it, decorate our homes with it or tap the touchscreens on our mobile phones and tablets. Glass is everywhere. It can be dangerous. When broken it can form shards that can inflict nasty injuries, while in its ornamental form it’s so delicate that an accident can mean a priceless artefact is shattered into thousands of pieces. Either way, glass is usually approached with an element of caution. But how did it come to hold these properties?
Before being manipulated, the components of this handy material are nothing but a pile of sand, rock and minerals. An unlikely combination of naturally occurring ingredients, when exposed to extreme heat they produce a fascinating reaction. Molten glass is the product’s middle stage, between sand grains and window panes. Baking in a fiery furnace, the red-hot liquid is unrecognisable compared to its final state.
At an atomic level, glass behaves in surprising ways at room temperature. Although it feels solid and is a hard substance to touch, scientists have discovered that glass never reaches a fully solid stage. The reason glass appears to be neither fully liquid nor solid is because it is structured more like a gel. When glass cools from being a searing, orange inferno, rather than crystallising and its atoms forming a lattice structure, it takes on a more random arrangement instead, creating a tight jam of particles. This makes the glass sturdy enough to appear solid without carrying all the properties of a solid.
As it is, glass is light, transparent and ideal for masses of applications. However, once turned from solid sand and rock to glass, it can’t be converted back. This makes glass difficult to recycle. To reuse glass, it can be melted back into its molten form, added to a new batch and reshaped for a different glass product. Recycling glass is vital to limit the natural resources humans use over time. If everybody put their glass bottles into the recycling bin, the glass already in circulation could be continuously reused without the need to make more. Theoretically this would mean having an eternal supply of glass, without having to use more of Earth’s raw materials.
Over 5,000 years have passed since people first explored a new substance that we now know as glass. The 21st century hasn’t failed to bring new uses for this flexible and widespread resource. New inventions bring new physical needs, while our expanding knowledge of science only widens the possibilities for a simple and ancient base material. As we evolve to develop technology further, who knows what is possible in the future of glass?
Making windows: from sand to sheet glass
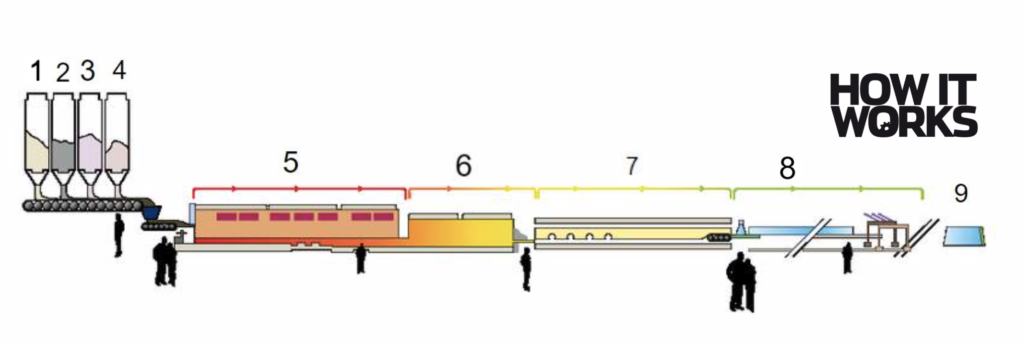
1. Silica sand
This is the primary ingredient, and the most common type of sand found in deserts. Consisting almost entirely of silica, the chemical purity of this material helps in making glass transparent.
2. Soda ash
Also known as sodium carbonate, this essential component reduces the melting temperature of the mixture.
3. Limestone
Found within rock, limestone adds some desirable physical properties to the finished product, such as durability and chemical resistance.
4. Sodium sulphate
This compound is added to the glass mixture to keep the quality consistently high. It acts to remove air bubbles from the liquid glass and keeps the substance smooth.
5. Melting
Once mixed, the raw materials enter a furnace. Faced with temperatures up to 1,500 degrees Celsius, the mixture melts.
6. Floating
This section is a float bath. When poured onto a bath of molten tin, the glass floats, keeping it flat at the surface. Turning gears keep the layer moving along the bath and can control the speed and thickness of the glass created.
7. Annealing
In this cooling phase, the liquid shrinks and begins to crystallise. This is usually a slow process to ease pressures gradually.
8. Inspecting
In between each stage, workers inspect the production line, ensuring the glass produced is of the highest quality. This inspection can also be automated, with machines scanning for missed bubbles and other inconsistencies.
9. Cutting
A machine at the end of the line cuts the glass to order. The edges of the glass sheets are trimmed for an even sheet and are sold by square metre.
For more science and technology articles, pick up the latest copy of How It Works from all good retailers or from our website now. If you have a tablet or smartphone, you can also download the digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!





