How to live to beyond 100

We are born, we live, we age and we die. This is the natural cycle of human existence, yet some people live longer than others. The world record holder for the longest human life is Jeanne Louise Calment of France, who lived to a magnificent 122 years and 164 days. But what is the secret to a long life?
Human beings are complex, and we live for a very long time, making studies of the process of ageing a serious challenge. Most of the research to date has therefore been done in animals. Two of the favourite species for these kinds of studies are Caenorhabditis elegans, a tiny worm about the size of this comma, and Mus musculus, the humble laboratory mouse. The worms generally live for just two or three weeks, while the mice have an upper lifespan of around three years, and both have a lot of genes that are quite similar to our own. Using these models, researchers have identified, several possible candidates, including stem cells, calorie restriction, and even some drugs, that could hold off the ageing process.
Scientists across the world have been trying to find the answers for decades, and after years of careful research, there is now a wealth of knowledge just waiting to be tested in people. We spoke to Brian Kennedy, CEO of the Buck Institute for Research on Aging: “We’re a non-profit medical research institute that’s focused on understanding ageing. We realised when the doors opened in 1999 that ageing was the biggest risk factor behind all of the diseases that we care about,” he explains. “I think the exciting thing that we have learned over the past decade is that it’s really possible to slow ageing in a mouse, or even in primates. The challenge now is to take that knowledge and apply it to humans. We’re not just talking about lifespan, what we really want to do is to extend healthspan: the period of time that you’re disease-free and functional. The field has amassed a whole load of candidates to slow ageing, and the challenge now is to figure out how to test them.”
Why do we age?
There is no easy answer to this question. As with almost everything else in biology, it is a combination of genetics and environment. One of the most well-established theories about why we age is that it is an accident of evolution. Charles Darwin’s famous theory explains that the ‘fittest’ or best-adapted animals will reproduce, passing on their genes to the next generation. To get this chance, they need to be able to survive through their early years, find a mate, and help their young to make it to adulthood.
Over the course of our lifetimes, our bodies take damage and start to deteriorate, but after reproduction, it doesn’t matter so much how long animals live. There is therefore much less pressure to evolve genes that extend life and reverse the damage. In fact, it might even be better in evolutionary terms to live fast and die young, if it means that you have a better chance of passing on your genes.
What makes us age?
There are several different factors thought to contribute to the ageing process
Damage
Over time, our DNA starts to accumulate mistakes. This is due to damage from the environment as well as errors made when our cells divide.
Calories
This is one of the most established areas of research. In mice, rats and even primates, limiting food intake to theminimum requirement extends lifespan.
Stem Cells
Stem cells can reproduce to replace cells that are damaged or worn out. As we age, they become less able to function, slowing the rate of repair.
Inflammation
Chronic inflammation is found in many age-related diseases, even when there is no infection to fight, but the relationship with ageing is unclear.
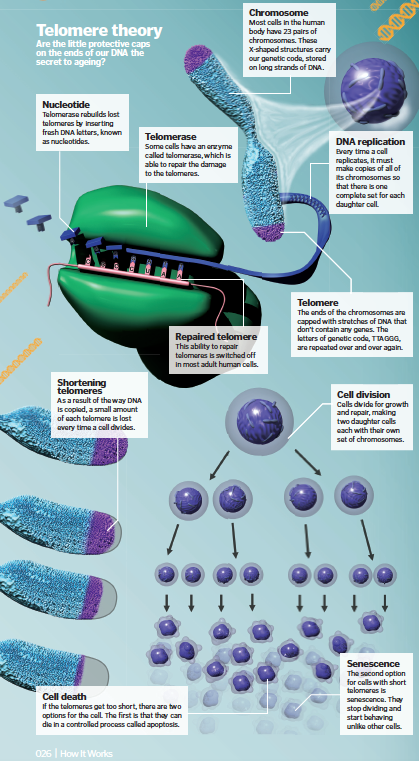
Telomeres
The ends of our chromosomes are capped with stretches of protective DNA called telomeres. Every time a cell divides, part of this cap is lost.
Glycation
Molecules called ‘advanced glycation end products’, or AGEs, form in our bodies over time. They have been implicated in several age-related diseases.
Do we have an age limit?
In 2010, an estimated eight percent of the world’s population was over the age of 65. By 2050, this is expected to rise to 16 per cent – that’s around 1.5 billion people. But despite this seemingly phenomenal increase in human lifespan, there has actually been little change in the upper limit of human age over the last 2,000 years.
Some people were living into their seventies back then, too. Brian Kennedy says: “Median life expectancy has been going up at a pretty high rate. But that’s median life expectancy. The question of whether we can extend the maximum is still a bit open.”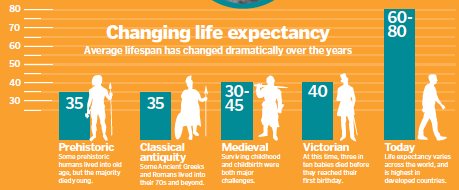
Almost all of our cells have 23 pairs of chromosomes. Each chromosome contains a long molecule of DNA, wound around a series of proteins to form an X-shape, and the ends are capped with structures known as telomeres. These have been a focus for anti-ageing researchers for many years because every time a cell divides, they get a little bit shorter.
Eventually, the telomere is so small that the cell can no longer go on dividing. As Professor Kennedy explains, “If you take cells out of the body and grow them in the test tube, it was found out many years ago that eventually, they stop growing. People have thought for 50 years now that this may be a component of ageing.” Telomeres can be lengthened again by an enzyme called telomerase, which is found in some stem cells. However, in most adult cells, telomerase is switched off. Without it, telomeres gradually get shorter as we get older, and our cells start to shut down. Some of these older cells die, while others just stop dividing and become ‘senescent’, which literally means ‘to grow old’. Researchers at the Buck Institute are very interested in senescence.
“One of our investigators, Judy Campisi, has been developing strategies to get rid of senescent cells in the body,” he continues. “The problem has always been that there aren’t that many senescent cells in the body, even in older people. It might be five percent of the tissue, ten percent of the tissue.” So the argument was always, ‘How can that have that big of an effect if it’s only a small proportion of the tissue?’ What Judy has found is that these senescent cells secrete factors that have bad effects on the cells in their environment.” Dr Campisi focused first on investigating the process in mice, and has developed a way to kill the senescent cells using genetic engineering. “When you do that, the animals stay healthy longer,” Kennedy explains. Dr Campisi is now working on finding a drug that can produce the same results. But the aim isn’t necessarily to extend life. These senescent cells could be contributing to age-related diseases, and that’s the real focus for the researchers. “Our goal is to keep people healthy and functional longer. They will probably live longer too, but it’s really about healthspan more than lifespan”.
To make sure you never miss an issue of How It Works magazine, subscribe today!





