Hot Air Balloon Physics: How do they fly?
by Scott Dutfield · 15/08/2019

How these big balloons rely on the principles of physics to fly
Hot air balloons were first invented in Paris in 1783 and were the first transport to allow humans to travel by air. Their design has remained similar over the centuries with a fabric ‘envelope’ to contain the hot air with a vent in the top and a basket underneath for the passengers.
Hot air balloons float due to the heated air inside the envelope having a lower density than the colder air outside. In the same way that a boat is supported by water on the ocean, it is cold air supporting a hot air balloon.
Operating a hot air balloon requires specialised knowledge and skill. A pilot must have an understanding of the wind directions at a different altitude, as the only method to steer is by catching these directional winds. By controlling the amount of hot air within the balloon a pilot can change the vertical direction either up or down.
For the vessel to be moved upwards, the pilot fires up a propane gas burner and can change the speed of ascent by controlling the strength of the flame. At the very top of the balloon is a self-sealing flap that can be controlled by the pilot by a long chord. This mechanism allows the pilot to let the hot air escape at a steady rate to either slow the ascent or cause the balloon to start descending back towards the ground. By moving the balloon up and down it is possible for the pilot to catch different winds moving in different directions to navigate the skies.
Floating physics
A Chinese lantern is a simple model for understanding the physics of hot air balloon flight
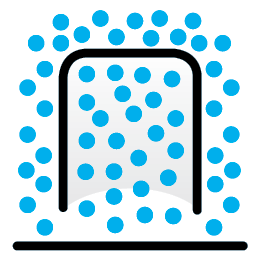
Uniform temperature
Without a flame, the air molecules inside and outside the lantern are the same temperature.
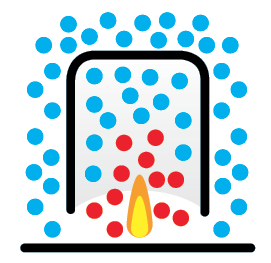
Flame
When the burner is lit within the lantern, chemical energy in the fuel is transferred to kinetic energy in the air.
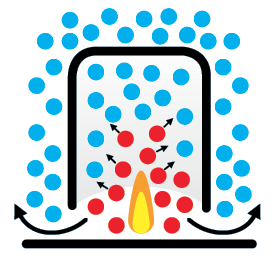
Heating up
The hot air molecules rapidly spread up and out, displacing the cold air molecules, which are either heated or ejected.
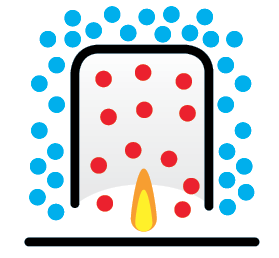
Temperature difference
The cold air molecules are pushed out of the lantern by the expanding volume of hot air.

Density
The expansion of hot air means it is less dense than the cold air, inflating the balloon and causing it to rise upward.
This article was originally published in How It Works issue 101, written by Charlie Evans
For more science and technology articles, pick up the latest copy of How It Works from all good retailers or from our website now. If you have a tablet or smartphone, you can also download the digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!




