How to make a giant salt crystal
by Scott Dutfield · 20/01/2021
Use the chemistry of crystallisation to form an enormous salt crystal at home
If you’re under 18, make sure you have an adult with you.

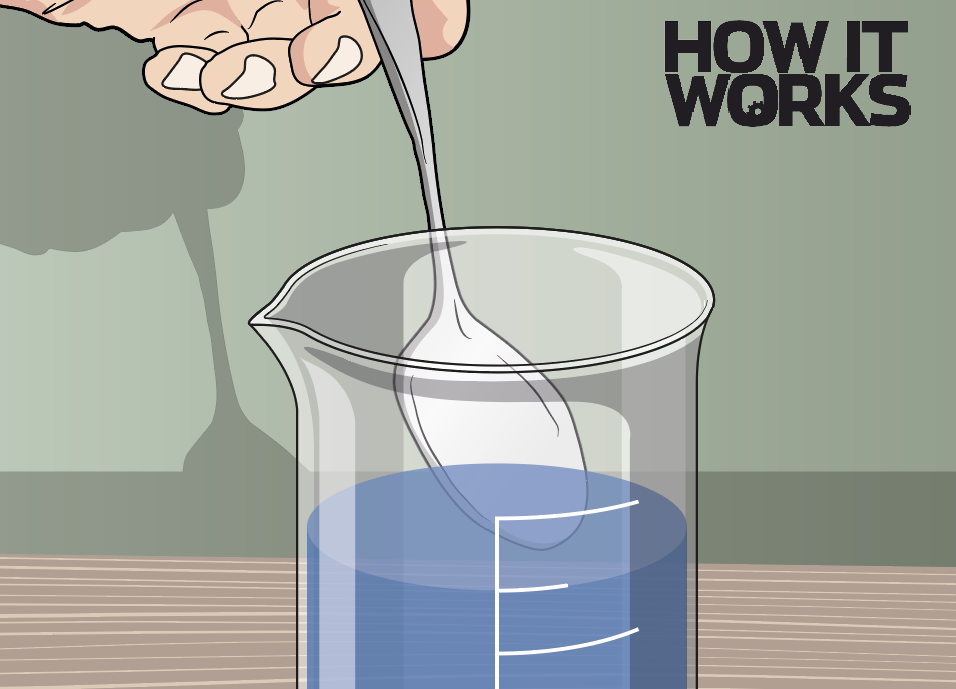
1. Dissolve the salt
First, ask an adult to heat around 500ml of water in a kettle. Pour it into a container, then add salt and stir it until it’s all dissolved. Keep adding salt to the water until it’s saturated.
2. Colour or clear?
If you want to make a coloured crystal, you can also add a few drops of food colouring to your liquid. Alternatively, you can leave it clear to make a white crystal.
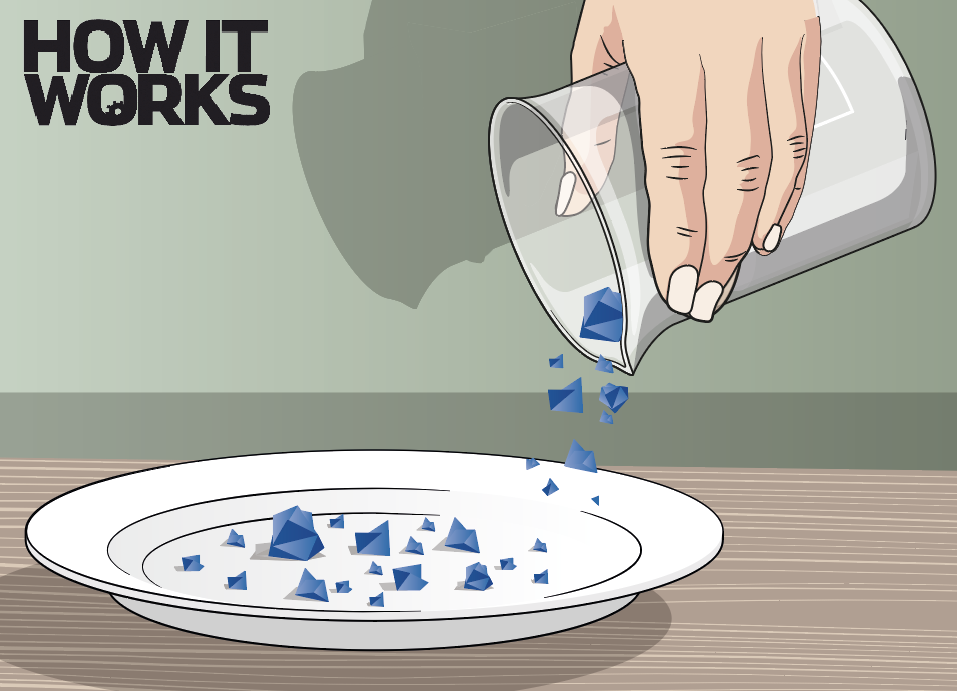
3. Find your crystal
Leave the mixture for a few days until crystals form at the bottom of the container. Pour the mixture into another container, then scrape out the crystals and choose a large, smooth one.

4. Filter and cool
Take the remaining solution and pour it through a filter to remove any impurities. Tie a string around the crystal you took out and hang it from a pencil so it rests in the solution.
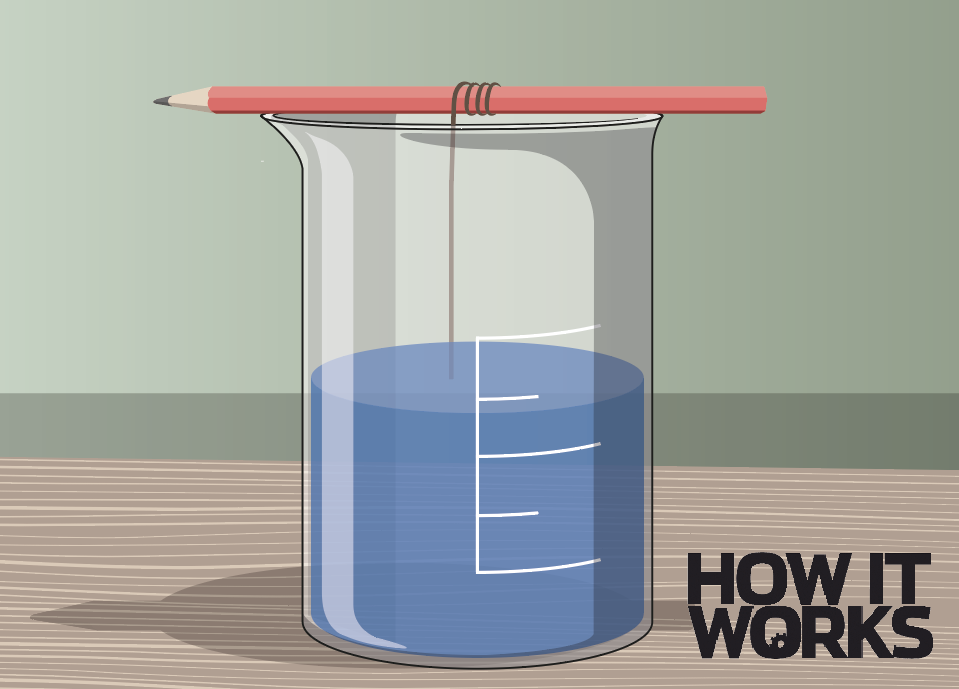
5. Start the process
Leave the mixture for a few days, and the crystal should start to grow as it attracts more salt particles from the solution. Try putting it in a cool place to speed things up.

6. Keep filtering
Every five days, take the crystal out and filter the solution again. This should remove any impurities in the water and ensure your crystal is pure salt.
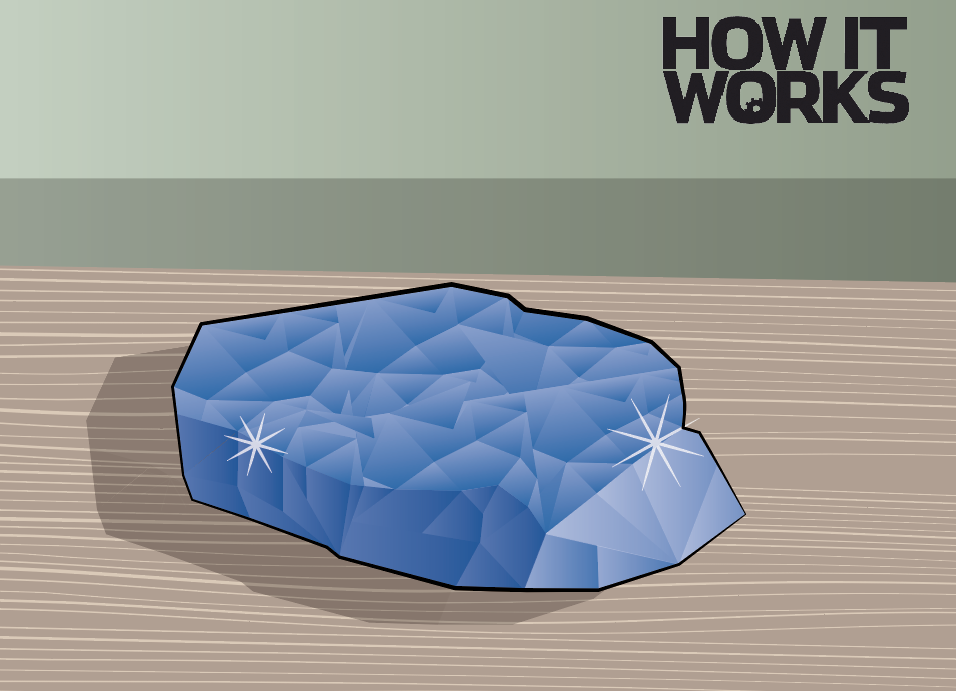
7. Wait it out
The longer you leave the solution, the larger your crystal will get. After around a month, you should have a crystal that is big enough to take out and display.

8. Polish your crystal
To preserve your crystal and avoid it absorbing moisture, paint the whole thing with transparent nail polish to create a protective barrier around the salt.
Summary…
When you dissolve table salt (sodium chloride) in water the sodium and chlorine atoms separate. Warm water can dissolve more salt than cool water, so when the water starts to cool, the two atoms reform into crystals. If there’s already a crystal in the solution, this attracts the newly formed atoms, making that crystal grow.
For more science and technology articles, pick up the latest copy of How It Works from all good retailers or from our website now. If you have a tablet or smartphone, you can also download the digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!




