Booze & Botanicals: How is gin made?
by Scott Dutfield · 17/01/2020

Botanicals to bottle, the science behind the UK’s favourite boozy beverage
Gin has been one of the nation’s favourite tipples since the early 1700s after it was introduced in London by the Dutch. The tantalising taste of this juniper-infused liquor rapidly grew in popularity and sent the residents of London into a period of gin-fuelled chaos around 1720, known as the ‘gin-craze’. Part of gin’s popularity was due to its accessibility and simple formula. The principle of creating basic gin is a matter of infusing the alcohol of a ‘base’ or neutral spirit with the aromatic flavours of juniper berries and other botanicals, such as coriander and lemon.
By distilling a combination of spirit, water and botanicals, the base is flavoured, and the final product’s Alcohol by Volume (ABV) percentage is dictated. The neutral spirit arrives at a distillery at around 96 per cent ABV, and after distilling the produced gin is cut to around 40 per cent ABV. The process to achieve this, however, varies between manufacturers to create their own unique gins.
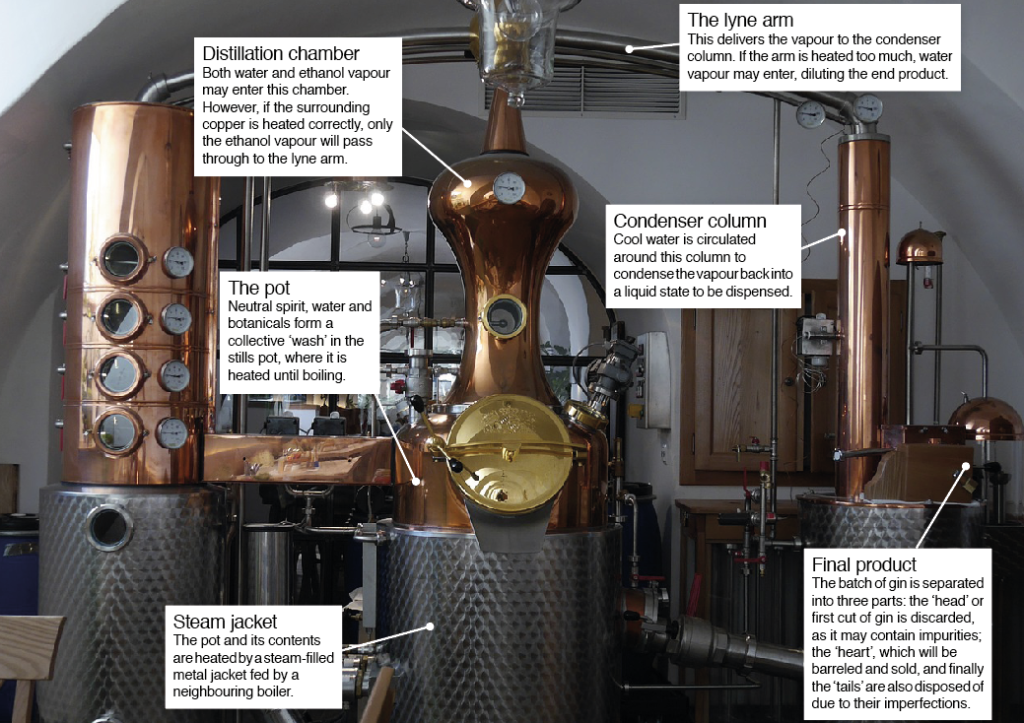
There are predominantly two methods to make this clear spirit, which can be scaled up or down for mass and domestic production. The first and more common utilises a large copper pot still whereby the neutral spirit is diluted with water and botanicals are steeped and heated for several hours. Some gin producers, however, suspend botanicals in copper baskets above the heated water and spirit wash for vapour infusion.
Once steeped, the mixture or ‘wash’ is then heated to produce an alcohol vapour that will travel up through connecting copper pipes. Differences in boiling points between both ethanol (around 78 degrees Celsius) and water (100 degrees Celsius), resulting in concentrated ethanol vapour escaping the wash to continue in the distillation process. Leaving the pot, this vapour flows to a condensation column, which facilitates the condensation of the vapour and is dispensed as gin.
The second method of gin distillation is similar to the first. However, rather than using a pot still for batch production, a continually filled column or ‘Coffey still’ is fed wash, heated before the condensed vapour is collected.
Pot distillation
How does a distillation process take gin from copper pot to cocktail glass
Click to expand
Created with copper
Notably, the prevailing material used to forge distilling apparatus for gin – and indeed a lot of other spirit distillations – is copper. This isn’t an industry choice based on aesthetics, but rather copper’s ability to benefit the final product’s overall taste. On a molecular level, copper reacts with the bitter-tasting sulphurs that are released from fermenting yeast during alcohol production. These sulphur molecules bind to the copper walls of the distillation chambers, forming copper sulphate, which is scrubbed away once the batch distillation is complete. Copper also helps create and maintain an even temperature as an enclosure for the heated gin wash within.
This article was originally published in How It Works issue 125
For more science and technology articles, pick up the latest copy of How It Works from all good retailers or from our website now. If you have a tablet or smartphone, you can also download the digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!




