How to make a fire snake
by Ailsa Harvey · 06/06/2020
Create a snake using sugar, baking soda and an amazing chemical reaction

DON’T DO IT ALONE: If you’re under 18, make sure you have an adult with you.
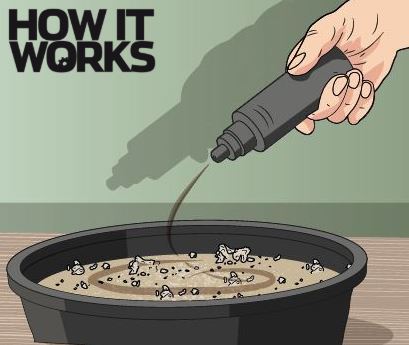
1. Create your insulator
To make your snake, first get a large bowl and then fill it with sand. Ask an adult to douse it in lighter fluid. Sand is fireproof and works very well as an insulator, so it should keep the bowl protected when things start to heat up.
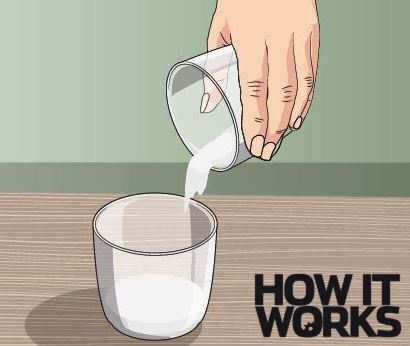
2. Prepare your mixture
The lighter fluid will act as fuel and helps maintain the heat of the flame for longer. Next you need to mix ten grams of baking soda (sodium bicarbonate) with 40 grams of sugar. Pour this mixture onto the sand.

3. Light it up
To light the mixture, you’ll need a long-necked match or a long-necked lighter. Ask an adult to help, and be very careful –the lighter fluid will ignite very quickly, so don’t get too close to the flame.

4. Snake attack!
After a little while, a large black blob will appear in the mixture and start to grow bigger. This is because as the baking soda and sugar get hot, they both release carbon dioxide gas, which begins to expand.

5. Carbon snake
The reaction also creates sodium carbonate, and as the carbon dioxide gas is created, the pressure pushes this upwards. The black colouring comes from the carbon in the mixture. It’s getting big now!

6. The snake's tail
After a while –probably around 20 minutes– the flames will start to go out and the snake will stop getting bigger. All of the baking soda and sugar have been used up at this stage, so there’s nothing left to burn.
(Illustrations by Ed Crooks)
Summary:
The chemical reaction in the bowl is activated by heat energy. When the mixture gets hot, the baking soda breaks down into sodium carbonate, carbon dioxide and water vapour. The sugar breaks down into carbon dioxide and water vapour too, adding more expanding gas and helping propel the carbon snake upwards.
Disclaimer: Neither Future Publishing nor its employees can accept any liability for any adverse effects experienced during the course of carrying out these projects or at any time after. Always take care when handling potentially hazardous equipment or when working with electronics and follow the manufacturer’s instructions.
This article was originally published in How It Works issue 130, written by Stephen Ashby
For more science and technology articles, pick up the latest copy of How It Works from all good retailers or from our website now. If you have a tablet or smartphone, you can also download the digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!





