Public swimming pools: what you don’t see
At first glance, a swimming pool might just look like a huge basin full of water, but hidden beneath the surface is a surprising amount of technology that keeps swimming pool water crystal clear.
The water is constantly circulated through a filtration system, passing out of the main pool through two or more main drains at the bottom, and ‘skimmer’ drains around the sides. The main drains collect any debris that sinks to the bottom of the pool, while the skimmer drains take in small amounts of surface water in order to sift out floating contamination, like hair and leaves.
The water is drawn through the system by an electric motor, which drives a pump. As the water flows towards the pump a sieve removes any large debris. The water then enters the filtration system, which contains high-grade sand in a vertical column. Gravity pulls the water through the sand, and small particulates become trapped in the tiny grains, allowing clean water to pass back out into the pool.
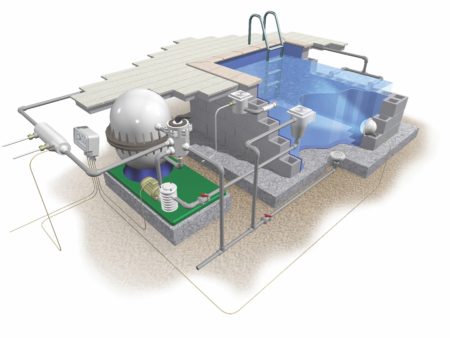
A sophisticated pump and filtration system keeps the water in your public swimming pool clean and clear.
The pump system generates powerful suction, which could create dangerous vortices in the water; the main drains have anti-vortex covers to prevent this from occurring. The use of multiple drainage points also minimises the risk of people becoming trapped by suction; if one drain becomes blocked, the pump draws water from the others, decreasing the suction and releasing the blockage.
A heater is often included in the pump system as well to warm the water as it passes through; a thermostat switches the heater on and off as required to maintain the temperature at a set level.
But why does the water taste funny?
The majority of the technology in a swimming pool is designed to keep it free of debris, and to maintain the water temperature. However, microscopic organisms thrive in warm, still water, and in order to keep the pool safe, chemical sterilisation is often used. Chlorine – either in the form of calcium hypochlorite or sodium hypochlorite – is added to the water as a disinfectant and gives it that distinctive taste and smell. It reacts with water to form hypochlorous acid, which interferes with bacterial cell walls, DNA and enzymes, preventing them from colonising the pool.
Hypochlorous acid breaks down when exposed to ultraviolet light, which is particularly problematic in outdoor swimming pools. Often a stabilising agent is added to keep the chlorine in a usable form for longer. Hypochlorous acid also reacts with ammonia (which is found in urine) to form compounds called chloramines, which smell bad and can irritate the eyes. A careful balance of chlorine and stabilisers must be maintained in order to keep the pool both safe and comfortable to swim in.
For more science and technology articles, pick up the latest copy of How It Works from all good retailers or from our website now. If you have a tablet or smartphone, you can also download the digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!




