How to freeze a glass of water instantly
by Scott Dutfield · 13/05/2021
Get the power of a superhero and freeze a glass of water with a single touch!
If you’re under 18, make sure you have an adult with you.
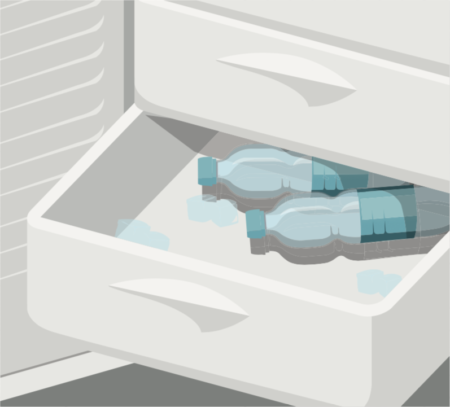
1. Cool your water
First, you need to freeze some purified water. You might think that you can make your own purified water for this experiment by boiling it for a few minutes, but that won’t remove the chemicals in the water, so you’ll need to buy specially purified water instead. Take three unopened 500ml plastic bottles of the water and place two of them in the freezer on their side.
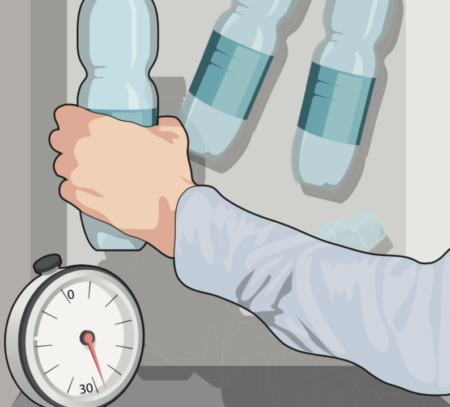
2. Be careful!
After 30 minutes put in a third bottle. Having more than one bottle will increase your chances of this working, so you can put in even more if you want to try this a few times! You need to leave your water in the freezer for two hours and 15 minutes in total. Make sure to leave the water as undisturbed as possible while it’s in your freezer, as agitation can start the crystallisation process.

3. Carefully remove it
After two hours and 15 minutes, slowly open the freezer and very carefully remove the lid of the bottle. If the process has worked correctly the water should still be liquid, but it will have been supercooled to below its freezing point. Tilt the glass you’re going to use and slowly pour the water into the glass. If you’re careful, the supercooled liquid shouldn’t start to solidify.

4. Grab some ice
You’ll need some crushed ice for this part. Put your finger into the crushed ice and make sure that there’s at least one ice crystal stuck to your fingertip. That’s all it will take to start the crystallisation process in the rest of the water. When you’ve got a crystal on your finger, gently lower your finger into the glass of supercooled water and watch what happens.

5. How did that happen?
If everything has worked properly the water should instantly start to solidify, with ice crystals spreading through the water to make ice. If you want to skip this step you can always just leave the water inside its plastic bottle and hit it on the side to kick-start the process. That one small movement is all that’s needed to start a chain reaction through all the molecules in the water!
In Summary…
Tap water will usually freeze at 0°C because of the chemicals and impurities in the water. The molecules can latch onto these impurities, and freezing is simple. In purified water there are no impurities, so if you’re careful the water can be cooled to well below its normal freezing point.
For more science and technology articles, pick up the latest copy of How It Works from all good retailers or from our website now. If you have a tablet or smartphone, you can also download the digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!





