The chemistry behind ice cream
From gelato to soft serve, what makes our favourite frozen treats?
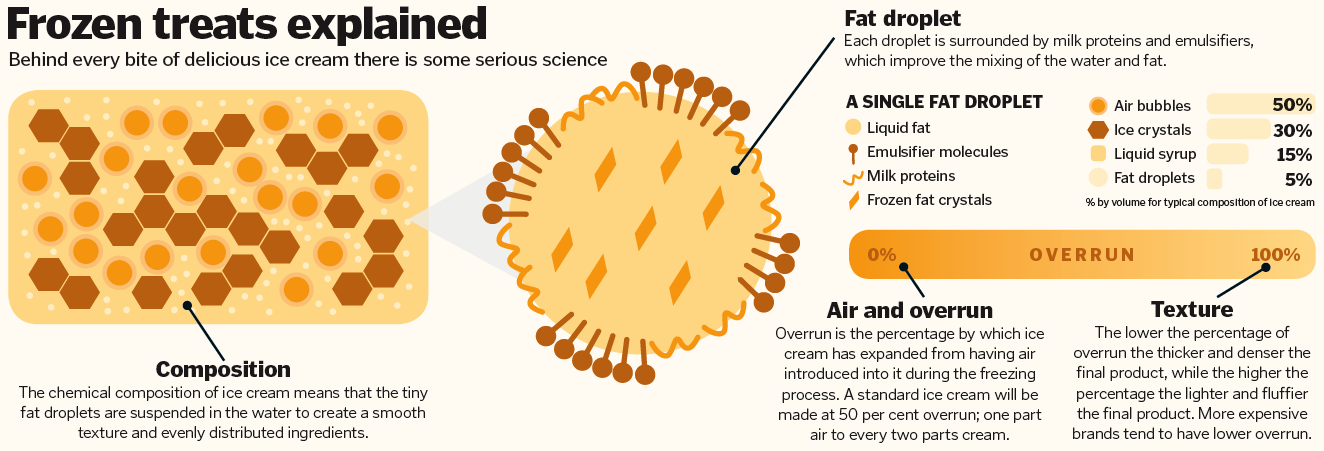
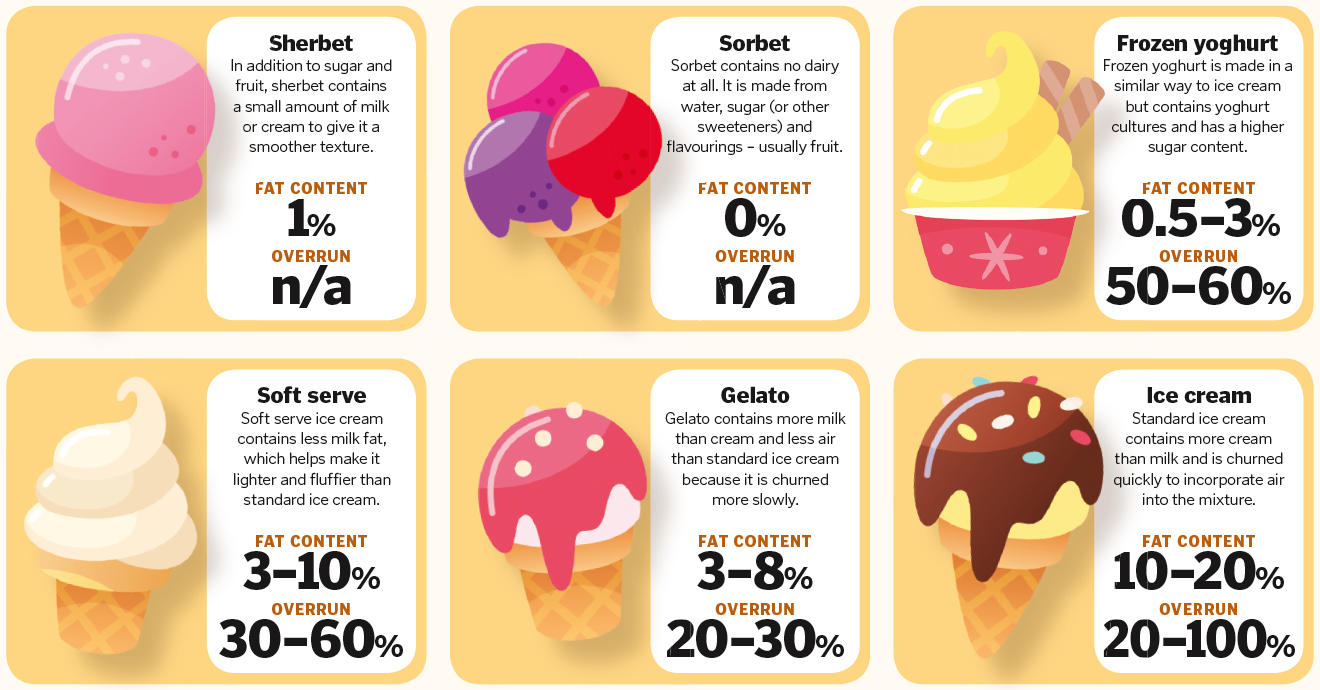
Ice cream is made from three main ingredients: milk, cream and sugar. However, you need to do more than just mix and freeze them to create the perfect dessert. Behind this seemingly simple summer treat, there is some complicated chemistry at play. Ice creams are an example of an emulsion; a combination of two liquids that would normally not mix together and are dispersed throughout each other. In ice cream, tiny droplets of fat are dispersed through the water. The fat comes from the cream, mostly in the form of triglycerides. During ice cream production they are aerosolised and broken down into tiny droplets. Milk proteins, which are also added during the production process, coat the fat droplets and prevent them from interacting with one another. This stops them from becoming large droplets again because the proteins stuck to the surface of the fat droplet repel one another. Many ice creams also contain emulsifiers, which surround the fat droplets and replace some of the milk proteins. This means that the droplets will mix more evenly when they are whipped. As the ice cream is frozen – usually with the help of liquid ammonia – it is also aerated, and the air becomes trapped in the desert by the arrangement of the fat, protein and emulsifying ingredients. The final and arguably most important ingredient is sugar. Not only does this make your ice cream taste great, it also lowers the freezing point of the water so you don’t end up with chunks of ice in your scoop. Add in some colourings and flavourings and your ice cream is ready to be enjoyed.
For more information about science and technology, visit our website now. If you have a tablet or smartphone, you can also download the latest digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!
Other articles you might like:
Coffee chemistry: Why does your morning hit of caffeine taste so good?





