12 experiments that changed the world

Cavendish weighs the world
Not only did the solitary and eccentric Henry Cavendish discover hydrogen, but he also successfully measured the weight of the world. His ambitious experiment used a special piece of equipment called a torsion balance, and in 1798 he reported his results. By measuring the gravitational attraction between two different sized lead spheres, he calculated the Earth’s density. The apparatus consisted of a 1.8-metre wooden rod that had a 0.73-kilogram lead sphere attached to each end, suspended from a wire. A separate system of two larger 159-kilogram lead balls were placed close to the smaller balls. This exerted enough gravitational force so that when the weights were tugged slightly the rod twists (a telescope was used to observe this). Cavendish performed his experiments in a dark and wind-proof to prevent any external air currents and temperature differences affecting his results. He was able to calculate the Earth’s density by using the ratios of the forces between the spheres and the gravitational attraction of the Earth to the spheres. Incredibly, his results were very accurate, and his great experiment meant we could also calculate the mass of the Sun and the Moon and even other planets in our Solar System.
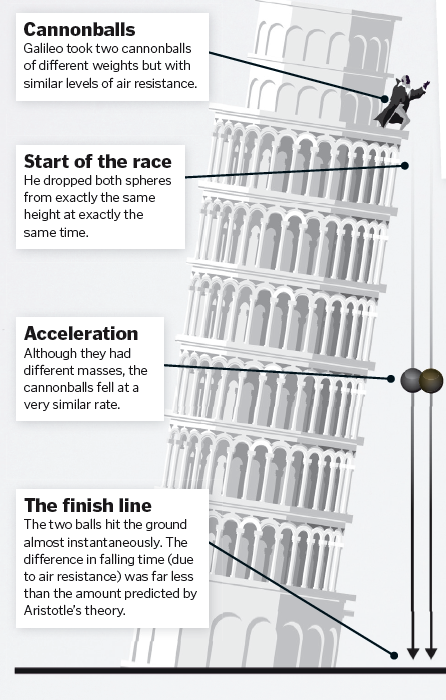
Galileo Galilei and the Leaning Tower of Pisa Experiment
Imagine you drop a bowling ball from one hand and a feather from the other. Which will fall faster? It is obviously the bowling ball, but this doesn’t reflect the nature of the force of gravity. Greek philosopher Aristotle had proposed that objects fell at different rates because gravity would act more strongly on heavier objects, but it turns out that the feather falls slower only because of air resistance. If you could perform the same experiment in a vacuum, the feather and ball will hit the ground at exactly the same time. It is difficult to separate fact from legend, but the story goes that Aristotle’s theory of gravity went unchallenged until Italian polymath Galileo Galilei disproved it. Though he spent the last years of his life imprisoned for going against the popular beliefs of the time, his work on speed, velocity, gravity and free fall provided the foundations of the understanding of how the planets and Solar System moved. Hundreds of years after his death his experiment was repeated on the Moon – unsurprisingly, Galileo was right
Mendel’s peas
How do we inherit our genes from our parents? The answer was actually discovered not by studying humans but peas. Gregor Johann Mendel, an Augustinian friar, crossbred peas with differing characteristics in order to evaluate how different features were inherited in their offspring. His work focused on pea plants and their seven observable traits: the shapes of the pods and seeds; plant height; flower position; and seed, pod and flower colour.
The study took around eight years, in which time he observed some 28,000 pea plants. When looking at the colour of peas produced, Mendel found that different generations of plants expressed different ratios of green and yellow peas, with yellow being the dominant colour. He discovered that genes are paired and the mathematical pattern seen throughout generations caused their dominant and recessive expression. This pattern can also apply to the genetics that code for our eye and hair colour.
Fleming’s accidental discovery of penicillin
1928, at St Mary’s Hospital in London, Alexander Fleming was busy investigating the bacterium, Staphylococcus aureus. The bacteria had been wreaking havoc, causing fatal infections, and there was no medicine at the time to treat them. On one occasion, Fleming forgot to put one of his Petri dishes into an incubator.
He noticed that a mould had a clear zone around it. He investigated and found that the mould had contaminated the dish, inhibiting the growth of the bacteria. England, 20th century While he was away on a two-week holiday the bacteria multiplied, and on his return he noticed something unusual in the rogue Petri dish. There was an area where the bacteria could not grow and instead left a ‘mould juice’ to form a clear Penicillium notatum. By the late 1930s, scientists Howard Florey and Ernst Boris Chain had managed to isolate and purify penicillin, and the antibiotic was available as an injection by 1941. It is estimated that this discovery has saved up to 200 million lives to date.
Pasteur uncovers the origin of cells
Back in the 1800s, people thought food spoiled and diseases were caused by ‘bad air’ or life spontaneously generating. Louis Pasteur didn’t – he rejected the idea that mice could be randomly created from rotting wheat and old cloth over a few weeks. After noticing that his own vats of beer were turning sour, Pasteur started analysing them only to discover they were swarming with bacteria. This convinced him that the spoiling of his brew was caused by these tiny microorganisms. He designed a simple experiment to prove his revolutionary germ theory, and as a result, disproved the idea that cells could come from nothing. So crucial was his work in the food and medicine industry that we even named a process after him – pasteurisation; the process of heat-treating something for a short time and cooling it down quickly to make it safe from bacteria.
Fermi’s nuclear reactor
After the atom was split and the term ‘nuclear fission’ was coined, physicist Enrico Fermi applied the principle to create the first self-sustaining nuclear chain reaction in a human-made reactor: Chicago Pile-1. Scientists were aware that a nuclear reactor would allow for the production of a weapon like nothing seen before. The outbreak of WWII meant that weapon production was a priority, a consequence of which was the birth of both the Manhattan Project and Fermi’s reactor. Once uranium-235 is hit with a neutron, the nucleus splits to form two smaller nuclei and more neutrons, which then go on to split other uranium atoms, thus forming a chain reaction. The reactor was made from stacks of graphite blocks to slow down fast uranium neutrons, increasing the likelihood of nuclear fission. This reaction needed to be controlled in order for it to be safe. Control rods made from cadmium were used to absorb the excess neutrons created from the nuclear fission. Adding or removing the rods could control the longevity of the chain reaction. This reaction produced large amounts of energy, which could then be harnessed for warfare.
Rutherford strikes gold
It was previously believed that the structure of the atom was a sphere of positive charge housing smaller negatively charged electrons within it, like plums within a pudding. To test the accuracy of this ‘plum pudding’ model — under the direction of Ernest Rutherford — Hans Geiger and Ernest Marsden performed a series of experiments between 1908–1913 to prove Rutherford’s theory of an atomic model, which resembled planets orbiting the Sun.
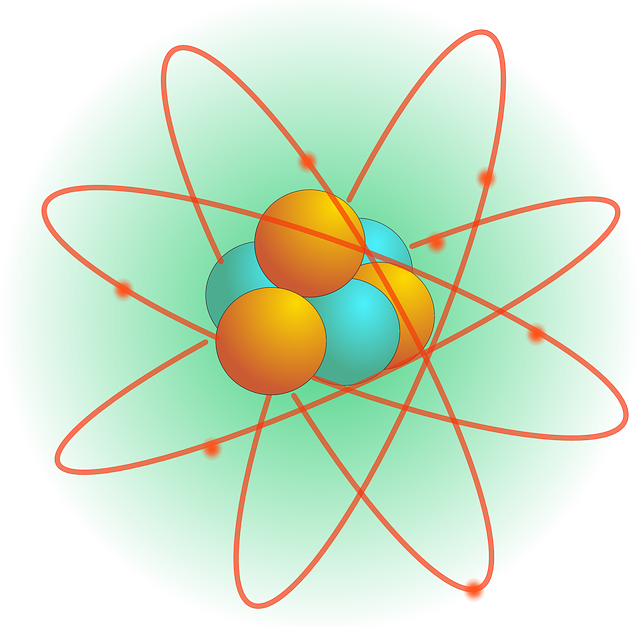
The physicists used a radioactive substance to bombard a thin piece of gold foil with positively charged alpha particles. The majority of particles passed through the foil without any deflection, suggesting that atoms had a great deal of open space.
However, some were deflected o the gold foil at different angles, which meant that those particular particles had hit something with the same charge. This meant that rather than a positive charge engulfing electrons, a smaller positive charge was held in the dense middle, thus heralding the discovery of the atomic nucleus.
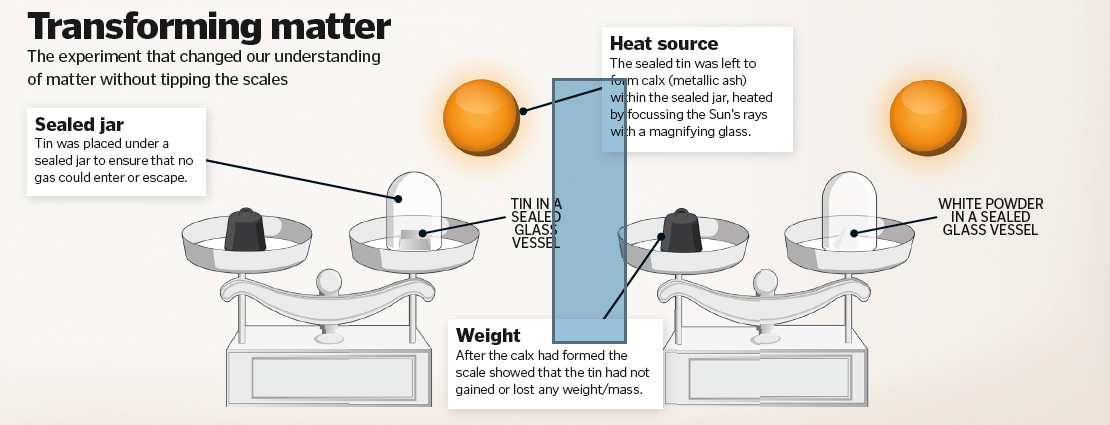
Lavoisier and the conservation of mass
It was a French chemist named Antoine Lavoisier who formulated the concept of the conservation of mass – the idea that matter can neither be created nor destroyed, only rearranged. He did so by measuring the mass of reactants and products during chemical reactions. One of Lavoisier’s experiments entailed placing a burning candle inside a sealed glass jar. As the wick burned down and the candle melted, the weight of the jar and its contents remained the same, thereby proving his pioneering theory. At the time, chemists were exploring the formation of calx (an oxide), predicting that metals lost mass as they were burnt. Lavoisier countered this with the idea that calx was the result of atmospheric gas interacting with the metal. Rather than the metal losing mass, he found it gained weight by combining with oxygen from the air.
Lind cures sailors’ scurvy
Bleeding gums, your teeth dropping out, weak limbs, swollen legs and nasty patches of blood under your skin – a pirate’s life probably wouldn’t have been ideal for most of us. Scurvy was one of the diseases that plagued pirates and sailors in the early days of seafaring. We know today that the disease is caused by a serious lack of vitamin C, something we need to form collagen, a vital component in structural and supportive connective tissue. Without enough collagen, the blood vessels and bones of those with scurvy break down until they suer a slow and painful death. But in the time of Scottish physician James Lind, there was no knowledge about these tiny nutrients. People thought that scurvy might be contagious or caused by madness. In 1747, Lind started one of the world’s first clinical trials. He suspected that acids could help stop the putrefaction of the body, and he devised a trial to test dierent ways of introducing certain acids into people’s diets. He divided a group of 12 scurvy-ridden sailors into six groups of two, all of which were to eat the same diet as one another but with the addition of an acidic supplement. Each group was treated with either a quart of cider, 25 drops of elixir of vitriol, two spoonfuls of vinegar, half a pint of seawater, two oranges and one lemon, or a spice paste, every day. After six days most of the sailors eating the fruit had made an almost complete recovery. While Lind was on the wrong track about the cause of the disease, he had found the cure.
Eddington and the eclipse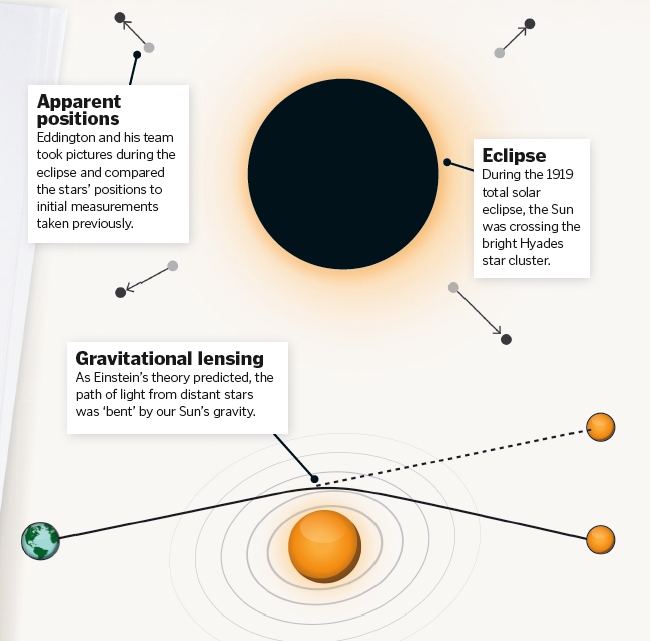
Scientific explanations in theoretical physics often remain just that, theoretical – but not all of them do. Albert Einstein published his general theory of relativity back in 1915, a criterion of which was that light bends near a massive gravitational force. However, Einstein was aware that should this or any of the other criteria required to support his revolutionary idea be disproven, then bang went the theory.
Einstein’s pioneering work remained a theory until an astronomer named Sir Arthur Eddington used an eclipse to prove light could be bent by gravitational forces. In order for Einstein’s theory to be correct, Eddington had to prove that the light had been bent by a source of intense gravity, such as the Sun.
A total solar eclipse in 1919 presented Eddington with a unique opportunity to witness the night sky during the daytime. After setting sail to Príncipe Island to get the best view of a predicted solar eclipse and test out Einstein’s theory, Eddington observed the locations of stars at night and then again under the false night of an eclipse. This meant that he could observe if the gravity of the Sun had altered the stars’ apparent positions, which in fact it had. This proved that light had been bent on its journey to Earth by way of the Sun’s gravity, meaning Einstein was correct.
The creation of graphene
In 2004, Professors Andre Geim and Konstantin Novoselov were experimenting with a graphite crystal. They removed some graphite flakes using sticky tape and, upon closer inspection, inspection, realised that some of the flakes were thinner than others. So they repeated the process, taking o more layers from the original peeled-o flake. Amazingly, their method worked. Each time the flakes were thinner, and they eventually managed to create flakes that were only one carbon atom thick. Although the existence of graphene had been predicted, no one knew how to isolate it. Until now. It sounds simple, but graphene has turned out to be a really important material and just what we needed in this digital age for display screens and electric/photonic circuits. A fantastic conductor of heat, dense, lightweight, flexible and transparent, it has been used in everything from tyres to transistor 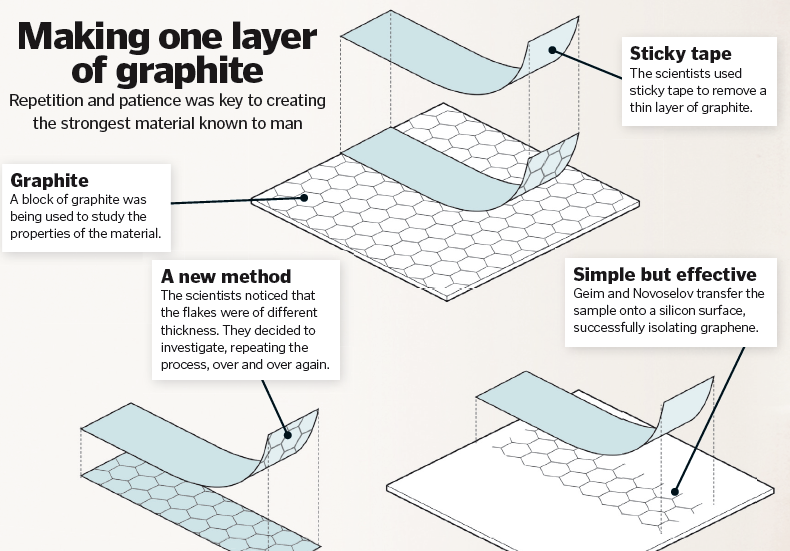
From theory to reality
In 1964, particle physicist Peter Higgs proposed a theory as to how particles have mass. He suggested that empty space is occupied by a field termed the Higgs field, where particles pass through it and either collect mass, like an electron or don’t interact with it at all and remain massless, such as a photon.
An analogy would be a person moving through a crowd of strangers versus a crowd of friends.
Moving through of crowd of strangers, you would pass easily without stopping, whereas surrounded by friends you might stop to talk, taking you longer to make your way through. In this case, your friends would be the Higgs boson. The Large Hadron Collider fires two beams of protons in opposite directions and accelerates them to near the speed of light so they collide to release the boson and other subatomic particles. This became a reality on 4 July 2012.
For more information about science and technology, visit our website now. If you have a tablet or smartphone, you can also download the latest digital version onto your iOS or Android device. To make sure you never miss an issue of How It Works magazine, subscribe today!




